Open Access | Case Report
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
The advantage of MRI in detection of Coincidental adenocarcinoma of palate and pleomorphic adenoma of parotid - a case report
*Corresponding author: Sleem, Heba Abdulwahed, Department
of Oral and Maxillofacial Surgery Ain Shams University, Cairo,
Egypt.
E-mail: drsleemh@gmail.com
Received: 15 August 2019 Accepted: 20 September 2019
DOI: 10.31491/CSRC.2019.09.004
Abstract
Salivary gland tumors account for fewer than 3% of the head and neck tumors. Typically salivary gland neoplasm presented as a single swelling localized to one salivary gland. A more unusual event is the development of multiple neoplasms located either in different glands or in one gland. This manuscript present a case of palatal swelling diagnosed by incisional biopsy as polymorphous adenocarcinoma (PAC). Magnetic resonance imaging (MRI) examination revealed another parotid lesion which was diagnosed as pleomorphic adenoma (PA). Palatal mass was resected with safety margin; superficial parotidectomy was also done for parotid lesion sparing facial nerve. Post-operative radiotherapy was recommended by pathologist due to evident perineural invasion of PAC. The patient is tumor free after one year follow-up. The present case has more than one risk factor which favors expectation of multiple tumors and mandate examination of all salivary tissues. In case of multiple salivary tumors each tumor should be diagnosed and treated separately according to its nature and extension.
Keywords
Salivary gland; tumors; parotid; palate; MRI
Introduction
Salivary gland tumors account for less than 3% of head
and neck tumors[1]. Many case reports describes presence of more than one salivary gland tumor either synchronously or metachronusly[2,3]. Several taxonomies
are used to describe synchronous/metachronous occurrence of salivary gland neoplasms. Multiple salivary tumors may be considered from three standpoints: according to incidence time (synchronous or metachronous ),
anatomical aspect (unilateral or bilateral), and histological aspect (identical or different pathology). Multiple
malignant salivary tumors of identical pathology are
much more common, which proposes the possibility of
genetic risk factor, rather than only accidental event[4].
Polymorphous adenocarcinoma (PAC) was first described in 1984, diagnoses is usually made based on
the hematoxylin and eosin morphology. Solid nests of
monotonous epithelial cells arranged in cribriform and
trabecular masses. Focal papillary cystic areas can typically be present within the same lesion. The epithelial
cells are usually cuboidal, columnar or spindle with eosinophilic cytoplasm. The nuclei are uniform, round or
ovoid, with a characteristic “washed out” appearance.
Mucous cells, clear cells or oncocytic cells may also be
seen. The concentric whorling is noticed around regional neurovascular bundles “a targetoid appearance”, this
perineural invasion (PNI) or neurotropism is a distinguishing feature of PAC and can be recognized in 30% of
cases. While perineural involvement is common, mitotic
figuresareuncommonandnecrosis is seeninhigh-grade
transformation[5].
PAC has a strong predilection to minor salivary glands of the
palate. It tends to have lower local recurrence rates and lower metastatic potential however, PAC is invasive and locally
destructive. Proper biopsy involving tumor periphery would
help definitive identification of the tumor. Invasive growth
at a lesion’s periphery, extension into non-neoplastic glands,
perineural and lymphovascular invasions all are pathognomonic features of PAC. Immunohistochemistry is mandatory
in absence of those diagnostic features. Long-term follow-up
surveillance is necessary because recurrences have been reported to occur more than 10 years after initial treatment[6].
Among all salivary gland tumors, pleomorphic adenoma
(PA) is the most commonly encountered tumor, it accounts
for about 60% of all salivary gland neoplasms. Parotid gland
(superficial lobe) is the most common extra-oral site while
palate is the most common intra-oral sit of PA. Pleomorphic
adenoma presented clinically as rubbery painless mass with slow growth rate[7]. Histologically, it is greatly variable in appearance hence the name pleomorphic adenoma. Characteristically it has a biphasic appearance, where a mixture of
polygonal epithelial cells and spindle-shaped myoepithelial
cells in a variable background stroma. Stroma could be cartilaginous, mucoid, myxoid or hyaline. Epithelial cells may be
arranged in duct-like structures, sheets, clumps or interlacing
cords. The tumor is not capsulated; however it is surrounded
by a fibrous pseudocapsule of variable thickness[8]. Surgical
resection with adequate safety margin is the main treatment
modality for salivary gland tumors. Underlying periosteal
layer should be included with or without bone resection according to nature of the lesion. Radiotherapy is indicated as an
adjuvant therapy if complete resection couldn’t be achieved,
to improve local recurrence. Generally, irradiation is reserved
for inoperable cases.
Co-occurrence of PAC and PA at the same time is very uncommon presentation with accidental detection during radiographic examination. Magnetic resonance imaging (MRI)
superiority in detection of concealed soft tissue tumors is the
key of diagnosis in such conditions. Perhaps adequate radiographic assessment for both minor and major salivary tissues
is mandatory to avoid miss management of such cases. This
work has been reported in line with SCARE 2018 [9].
Case Report
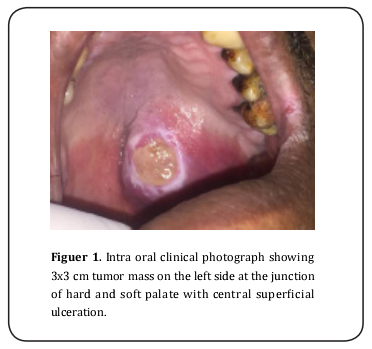
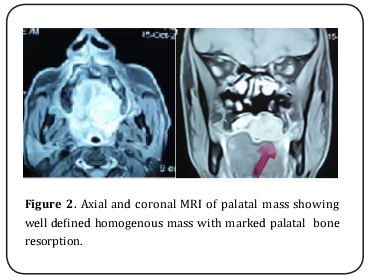
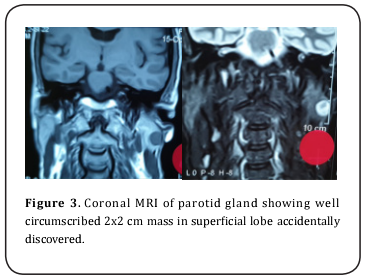
A 52-year-old male patient visited the maxillofacial surgery outpatient clinic attached to AIN Shams University, complaining of palatal painless swelling over a period of two months. Past medical and surgical history was non-contributory. Social history was significant for 30 years of smoking. No significant family history was reported. Intra -oral clinical examination revealed 3x2x2.5cm rubbery well defined mass on the left side of the palate. Covering mucosa had normal looking with small surface ulceration 1x1cm (Figure 1). Intra-oral examination for other abnormalities was unremarkable. Clinical examination of related lymph nodes and other salivary glands fail to discover any positive finding. At this point minor salivary gland neoplasm was the preliminary diagnosis and our patient was scheduled for a diagnostic incisional biopsy. Meanwhile magnetic resonance imaging (MRI) examination of the head and neck was requested, the imaging showed 3x3x2cm heterogeneous soft tissue mass occupying the left maxilla crossing the midline with cortical impingement of underlying bone (Figure 2). Another well-defined homogenous circular mass 2x2 cm in superficial lobe of ipsilateral parotid gland was accidentally discovered, however no positive lymph nodes detected (Figure 3).
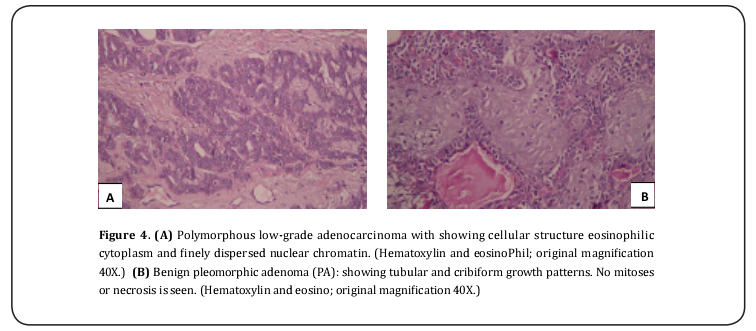
Incisional biopsy under local anesthesia was done for diagnosis of palatal swelling. Pathology report diagnose the palatal tumor as “polymorphous adenocarcinoma (PAC). Ultra sound (US) guided fine-needle aspiration cytology (FNAC) of parotid mass was done showing a histopathological picture suggestive of pleomorphic adenoma (Figure 4). Work up was done to exclude other primary or synchronous tumors including chest computed tomography (CT) and abdominal ultrasound.
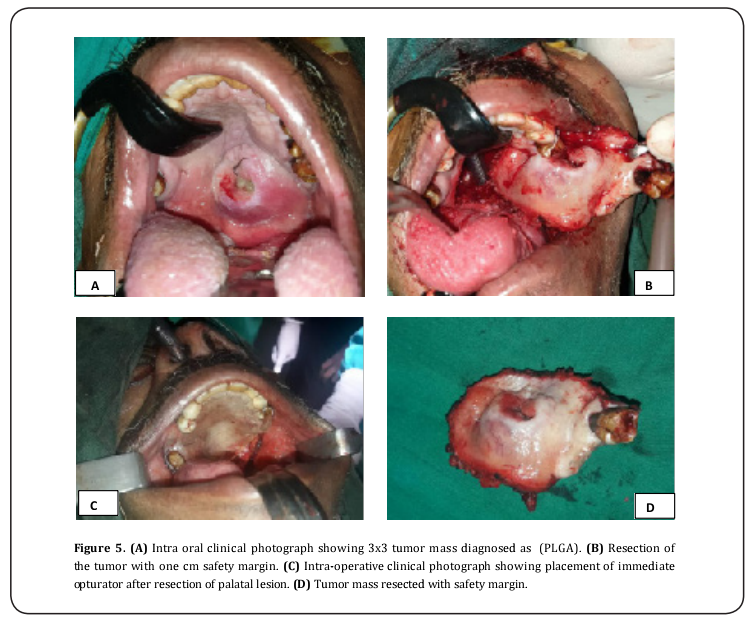
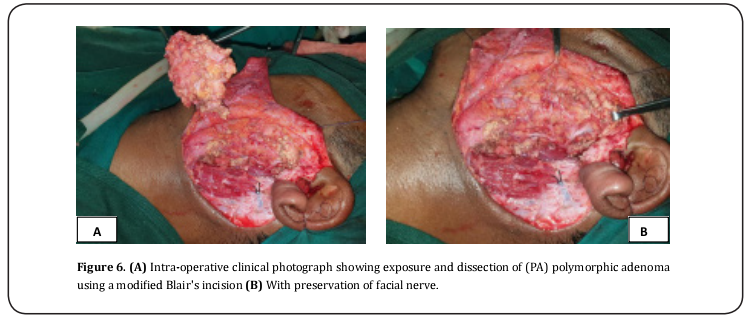
The decision was made to surgically resect the palatal tumor with one centimeter safety margin including the underlying palatine bone and insertion of immediate obturator (Figure 5). For synchronously discovered parotid tumor superficial parotidectomy with facial nerve preservation was done through modified Blair incision followed by primary closure (Figure 6). Operative time was five hours with blood loss about 250-350mm, no intra or post-operative complications were recorded. Patient was discharged two days after operation. Treatment plane was explained to the patient to ensure his acceptance before obtaining informed consent. Resected palatal mass showed perineural invasion (as testified by pathology report) accordingly the patient was scheduled for postoperative radiotherapy to control perineural invasion of greater palatine nerve. External beam radiation 60Gy five days per week for six weeks was the recommended protocol by radiotherapist. Patient was free from recurrence after follow up for one year with intact facial nerve function. Two weeks after first radiation dose the patient complains of xerostomia, sore throat, burning mouth, hoarseness, dysphagia, pain, and marked limitation in mouth opening. However, these side effects diminished after two months except for xerostomia.
Discussion
Although salivary gland neoplasms remain idiopathic, there
are many risk factors reported in literature including radiation, genetic predilection, excessive smoking, certain oncogenic viral infections and exposure to certain chemicals.
Excessive exposure to diagnostic or therapeutic radiation has
considerable increased risk of salivary gland neoplasia. There
is also significant risk for carcinoma of other organs secondary
to primary benign or malignant salivary tumors, especially
breast, thyroid, bronchus and ovary[10-12]. Polymorphous adenocarcinoma (PAC) were the most reported minor salivary
tumor in cases of multiple salivary neoplasms. The mean age
for patients with multiple malignant salivary gland neoplasms
at the time of their first primary tumor was 53 years[13]. Both
findings are consistent with our case report.
In cases of multiple salivary tumors pathology of each lesion
should be addressed separately. If histopathology is similar
the possibility of there being one primary tumor with distant
metastasis should be considered[3]. In case of different pathology each tumor should be treated independently according to
location, size, anatomical extension as well as histological phenotype[4]. Recently PAC has been differentiated from a new
pathologic variant of salivary tumor named mammary analog
secretory carcinoma (MASC). The present case showed clear
microscopic features of PAC, in addition there is no conclusive evidence that (MASC) should be treated differently than
any other low-grade malignant salivary gland tumors, though high-grade transformation has been described[14].
Radiological examination is mandatory in salivary tumors
workup. Although ultra sound US is useful in imaging of
superficial masses it neither detects malignant features nor
invasion of surrounding tissues[13]. The next step in diagnosis
is biopsy; FNAC is most commonly adopted technique for
diagnosis of parotid tumors. It is a quick and safe sampling
technique that can be performed readily in the outpatient
setting with high specificity and diagnostic accuracy (89%
and 85%). However it is technique sensitive. Sensitivity for
detecting malignancy has been reported between 70% and
80%. The diagnostic accuracy can be increased significantly when used in conjunction with a cytology and Image guidance. In the present case US was used to guide fine needle
aspiration cytology (FNAC) to overcome sampling error[15,16].
CT with contrast is the most commonly used investigation in
diagnosis of salivary tumors. In fact MRI is superior in early
detection of soft tissue changes and characterization of malignant nodal involvement, detection of underlying bone marrow infiltration as well as anticipation of perineural spread[17].
Lymph node involvement in MRI is specified by two criteria:
size larger than one centimeter with loss of central fat (central necrosis). The use of MRI rather CT in the present case
allows clear detection of coincident parotid lesion that could
be missed by CT. None of the examined lymph-nodes showed
such criteria accordingly we adopt wait and see policy with
frequent clinical and radiographic follow-ups[18,19].
In the present case both lesions were surgically managed in the
same operation. Minor salivary tumor PAC was resected with
one cm safety margin including underlying palatal bone to
ensure tumor free margins as recommended by literature[13].
Insertion of immediate opturator after resection of PAC was
decided since it allows frequent examination of the surgical
bed for detection of tumor recurrence. Superficial parotidectomy with facial nerve dissection and preservation was
the treatment of choice for benign PA[6]. It has been reported
in literature that simple enucleation of PA is associated with
high recurrence rates (8% and 45%), which is reduced to less than 5% with superficial parotidectomy and 0.4% with total
parotidectomy. this was attributed to presence of microscopic
extensions protruding beyond pseudocapsule or by capsular
penetration[20,21]. Unfortunately histopathological evidence of
perineural invasion of PAC (through greater palatine nerve)
mandate radiation therapy which adds to patient disability.
Kämmerer et al, in 2009 report a case of polymorphous lowgrade adenocarcinoma misdiagnosed by incisional biopsy as
PA, excisional biopsy later on revealed areas of small malignant fractions of the specimen besides a major part of benign
tissue formations. Our case could be a logic explanation of
such contribution. Where palatal tumor started as PA and
transformed gradually into PAC. By this explanation both
tumor would have same pathology at initial presentation[22].
We aim to report a rare incidence of multiple primary synchronous salivary tumors of different pathology. Where, the
use MRI in diagnosis of salivary tumors is mandatory to detect
even early small coincident salivary tissue involvements.
Conclusions
The use of MRI examination is mandatory for exclusion of multiple salivary tissue involvement especially if patient has risk factors for malignancy (e.g.: smoking, old age).
Ethical approval
Ethical approval has been exempted by faculty ethical committee based on absence of any risk or violation of applied guidelines in such cases.
Conflicts Of Interest
All authors declared that there are no conflicts of interest.
References
1. Licitra, L. , Grandi, C. , Prott, F. J. , Schornagel, J. H. , Bruzzi,
P. , & Molinari, R. . (2003). Major and minor salivary glands
tumours. Critical Reviews in Oncology/hematology,
45(2), 215-225.
2. Clayton, J. R. , Pogrel, M. A. , & Regezi, J. A. . (1995).
Simultaneous multifocal polymorphous low-grade
adenocarcinoma. report of two cases. Oral Surgery Oral
Medicine Oral Pathology Oral Radiology & Endodontology,
80(1), 71-77.
3. Luz María Ruíz-Godoy R, Mosqueda-Taylor, A. , Lourdes
Suárez-Roa, Poitevin, A. , Esther Bandala-Sánchez, &
Abelardo Meneses-García. (2003). Hybrid tumours of the
salivary glands. a report of two cases involving the palate
and a review of the literature. European Archives of OtoRhino-Laryngology, 260(6), 312-315.
4. Seifert, G. , & Donath, K. . (1996). Multiple tumours of
the salivary glands—terminology and nomenclature.
European Journal of Cancer Part B Oral Oncology, 32(1),
0-7.
5. Turk, A. T. , & Wenig, B. M. . (2014). Pitfalls in the
biopsy diagnosis of intraoral minor salivary gland
neoplasms. Advances In Anatomic Pathology, 21(1), 1-11.
6. Vincent, S. D. , Hammond, H. L. , & Finkelstein, M. W. . (1994).
Clinical and therapeutic features of polymorphous lowgrade adenocarcinoma. Oral Surgery Oral Medicine & Oral
Pathology, 77(1), 41.
7. Johnson, J. T. , Ferlito, A. , Fagan, J. J. , Bradley, P. J. , & Rinaldo,
A. . (2007). Role of limited parotidectomy in management
of pleomorphic adenoma. The Journal of Laryngology &
Otology, 121(12), 1126-1128.
8. Ito, F. A. , Jorge, J. , Vargas, P. A. , & Lopes, M. A. . (2009).
Histopathological findings of pleomorphic adenomas of
the salivary glands. Medicina Oral Patología Oral Y Cirugía
Bucal, 14(2), E57.
9. Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A.,
Orgill D.P. .(2018).The SCARE 2018 statement: Updating
consensus Surgical CAse REport (SCARE) guidelines.
International Journal of Surgery,60,132-136.
10. Auclair, P. L. (1991). Salivary gland neoplasms: general
considerations. Surgical pathology of the salivary glands,
135-164.
11. Schneider, A. B., Favus, M. J., Stachura, M. E., Arnold, M. J.,
& Frohman, L. A. (1977). Salivary gland neoplasms as a
late consequence of head and neck irradiation.Ann Intern
Med, 87(2), 160-4.
12. Horn-Ross, P. L., Ljung, B. M., & Morrow, M. (1997).
Environmental factors and the risk of salivary gland
cancer. Epidemiology, 414-419.
13. Clayton, J. R., Pogrel, M. A., & Regezi, J. A. (1995).
Simultaneous multifocal polymorphous low-grade
adenocarcinoma: Report of two cases. Oral Surgery,
Oral Medicine, Oral Pathology, Oral Radiology, and
Endodontology, 80(1), 71-77.
14. Boliere, C., Murphy, J., Qaisi, M., Manosca, F., & Fung, H.
(2019). Mammary Analogue Secretory Carcinoma of the
Palate: Case Report and Review of the Literature. Case
reports in dentistry, 2019.
15. Orell, S. R. (1995). Diagnostic difficulties in the
interpretation of fine needle aspirates of salivary gland
lesions: the problem revisited. Cytopathology, 6(5), 285-
300.
16. Haldar, S., Sinnott, J. D., Tekeli, K. M., Turner, S. S., & Howlett,
D. C. (2016). Biopsy of parotid masses: review of current
techniques. World journal of radiology, 8(5), 501.
17. Lee, Y. Y. P., Wong, K. T., King, A. D., & Ahuja, A. T. (2008).
Imaging of salivary gland tumours. European journal of
radiology, 66(3), 419-436.
18. van den Brekel, M. W. (2000). Lymph node metastases: CT
and MRI. European journal of radiology, 33(3), 230-238.
19. Moonis, G. , Patel, P. , Koshkareva, Y. , Newman, J. , &
Loevner, L. A. . (2007). Imaging characteristics of recurrent
pleomorphic adenoma of the parotid gland. Ajnr Am J
Neuroradiol, 28(8), 1532-1536.
20. Park, S. Y., Han, K. T., Kim, M. C., & Lim, J. S. (2016). Recurrent pleomorphic adenoma of the parotid gland. Archives of
Craniofacial Surgery, 17(2), 90-92.
21. Zbären P. , Poorten, V. V. , Witt, R. L. , Woolgar, J. A. , Shaha,
A. R. , & Triantafyllou, A. , et al. (2013). Pleomorphic
adenoma of the parotid: formal parotidectomy or limited
surgery?. American journal of surgery, 205(1),108-119.
22. Kämmerer, P. W., Kreft, A., Toyoshima, T., Al-Nawas, B.,
& Klein, M. O. (2009). Misleading initial histological
diagnosis of a polymorphous low-grade adenocarcinoma
in situ ex pleomorphic adenoma—a case report. Oral and
maxillofacial surgery, 13(2), 99-103.