Open Access | Research Article
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Prognostic and Clinicopathological Significance of Programmed Death-Ligand 1 in Osteosarcoma: a Meta-Analysis
*Corresponding author: Xiao-Bin Gu,Department of Radiation Oncology, The First
Affiliated Hospital of Zhengzhou University, No. 1 Jianshe East
road, Zhengzhou 450052, Henan Province, China.
E-mail: fahzzugu@126.com
Received: 20 April 2019 Accepted: 22 June 2019
DOI: 10.31491/CSRC.2019.06.031
Abstract
Background: Programmed cell death-ligand 1 (PD-L1) was reported to be associated with survival outcomes in patients with osteosarcoma, but the results were controversial. This study aimed to evaluate the prognostic value of PD-L1 in osteosarcoma.
Methods: The pooled HR and 95%CI were calculated to measure the prognostic role of PD-L1 for overall survival (OS) and disease-free survival (DFS). The odds ratio (OR) and 95%CI were used to evaluate the correlation of PD-L1 and clinicopathological features. Publication bias was measured using Begg’s funnel plots. Results: A total of 9 studies with 538 patients were included in this meta-analysis. The pooled results were HR=1.78, 95%CI=1.36-2.32, p > 0.001 for OS and HR=1.24, 95%CI=0.31-5.07, p=0.761 for DFS. PD-L1 was significantly associated with metastasis (OR=8.51, 95%CI=4.3-16.86, p > 0.001).
Results: A total of 9 studies with 538 patients were included in this meta-analysis. The pooled results were HR=1.78, 95%CI=1.36-2.32, p<0.001 for OS and HR=1.24, 95%CI=0.31-5.07, p=0.761 for DFS. PD-L1 was significantly associated with metastasis (OR=8.51, 95%CI=4.3-16.86, p<0.001).
Conclusion: PD-L1 might be a potential prognostic marker in patients with osteosarcoma.
Keywords
meta-analysis; PD-L1; osteosarcoma; prognosis
Introduction
Osteosarcoma is the most common cancer type of bone
originating sarcoma and mainly occurs in adolescents
and young adults [1]. Osteosarcoma accounts for approximately 20% of all primary bone tumors [2]. The
treatment strategies of osteosarcoma vary in different
stages. Surgical resection is the main treatment method
for primary tumor and distant metastasis often occurred
after surgery. About 40% of osteosarcoma patients are
in advanced stage at first diagnosis[3]. Only two-thirds
of patients of localized stage are expected to be cured.
Novel prognostic markers for osteosarcoma patients are
needed to guide clinical management.
As an important mechanism for immune activation and
suppression, the programmed cell death 1 (PD-1) and
programmed cell death-ligand 1 (PD-L1) pathway attractive much attention [4]. PD-L1 is expressed in specific
tumors and T and B cells, macrophages [5]. The combination of PD-L1 and PD-1 leads to the suppression of
the proliferation and effective responses of T cells [5].
Previous studies showed the prognostic significance of
PD-L1 in various solid tumors including colorectal cancer [6], breast cancer [7], prostate cancer [8], hepatocellular
carcinoma [9], and ovarian carcinoma [10]. Recent studies
also investigated the prognostic value of PD-L1 in osteosarcoma [11-15], whereas the results are inconsistent.
Therefore, I performed a meta-analysis to clarify the
prognostic and clinical significance of PD-L1 in patients
with osteosarcoma.
Materials and Methods
Search strategy
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement[16]. The ethical approval is not required because all patients are anonymous, and data were collected from the published literature. The databased of PubMed and Web of Science were searched using the following search strategy: (“Programmed Cell Death Ligand 1” or “Programmed Death Ligand 1” or “PDL1” or “B7-H1” or “CD274”) and (“osteosarcoma” or “osteogenic sarcoma”). The last search was updated to May 2019. Moreover, the reference lists were also checked for potentially relevant studies.
Selection criteria
The inclusion criteria for eligible studies were as follows:
1) the diagnosis of osteosarcoma was histologically
confirmed; 2) the expression of PD-L1 was detected by
immunohistochemistry (IHC) or reverse-transcriptase
PCR (RT-PCR); 3) PD-L1 was divided as high and low
groups; 4) the relationship of PD-L1 and survival and/or clinical factors were investigated, and the relevant
data were provided or hazard ratio (HR) and 95% confidence intervals (CIs) and be calculated [17]; 5) published
in English.
Studies were excluded by the following criteria: 1) reviews, meeting abstract, case reports, or letters; 2) duplicated or overlapping studies; 3) animal studies.
Data extraction and quality assessment
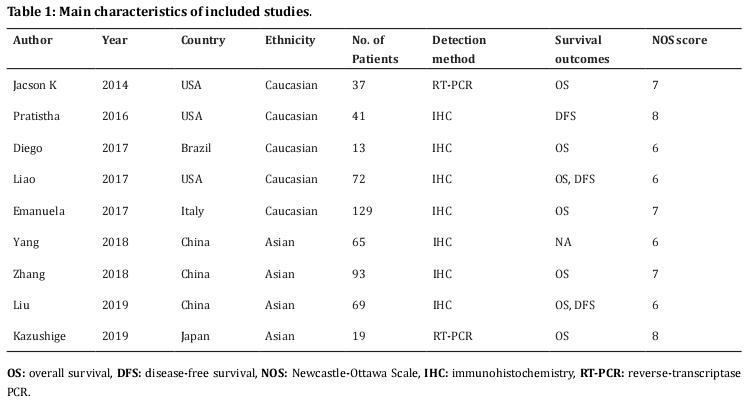
The following information were extracted from eligible studies according to a standardized data-collection protocol: name of the first author, year of publication, study location, sample size, ethnicity, detection method, survival endpoints, HRs and 95%CIs for overall survival (OS) and disease-free survival (DFS). Quality assessment was conducted by using Newcastle-Ottawa Scale (NOS)[18]. The maximum score of NOS is 9 points and studies scoring greater than six considered to be of high quality.
Statistical analysis
The pooled HR and 95%CI were calculated to measure the prognostic role of PD-L1 for OS and DFS. Statistical heterogeneity among studies was evaluated by Cochran Q test [19] and I2 statistic [20]. I2 > 50% or P < 0.10 was considered as significant heterogeneity, and the random-effect model was used, otherwise, a fixed-effect model was applied. The odds ratio (OR) and 95%CI were used to evaluate the correlation of PD-L1 and clinicopathological features. Subgroup analysis was performed for further investigation. Publication bias was measured using Begg’s funnel plots [21]. The statistical analysis was conducted using Stata version 12.0 (Stata Corporation; College Station, TX, USA). A P value < 0.05 was considered as statistically significant.
Results
Literature selection and characteristics
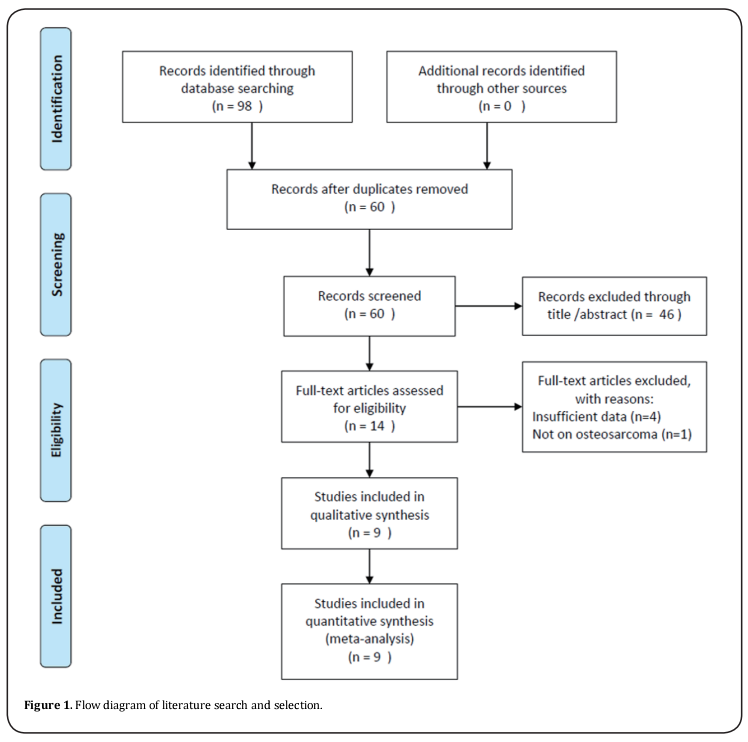
A total of 98 studies were identified through initial literature search. After duplicate studies were excluded, 60 records were examined by title and/or abstract. The 46 studies were removed by title and/or abstract screening and 14 studies remained for full-text assessment. After full-text inspection, 6 studies were excluded because of insufficient data (n=5) or not on osteosarcoma (n=1). At last, 9 studies [11-15,22-25] were included in this meta-analysis. The selection process is shown in Figure 1. The included studies published from 2014 to 2019. Three studies were conducted in USA [11,12,14], three in China [22-24], and one in Brazil [13], Italy [15] and Japan [25], respectively. The total sample size was 538, ranging from 13 to 129. All studies were retrospective studies and published in English. The NOS score ranged from 6 to 8, indicating that all eligible studies were of high quality. The main characteristics of included studies were shown in Table 1.
PD-L1 and OS, DFS
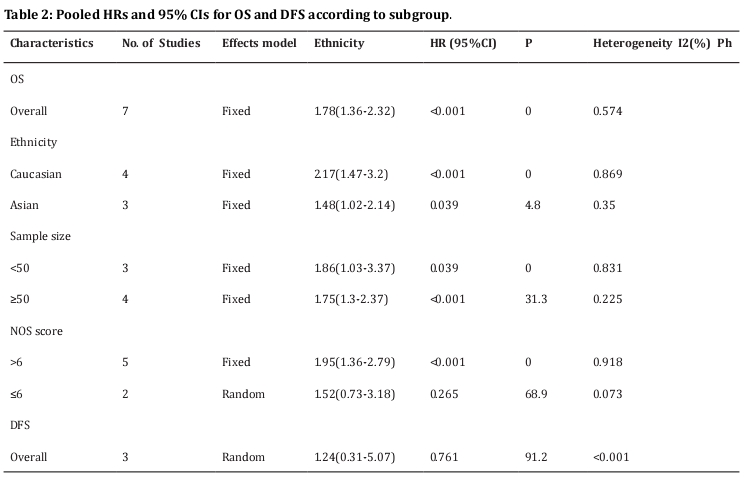
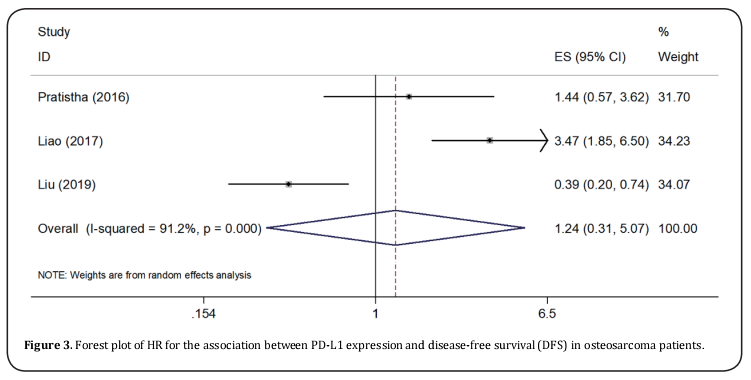
Seven studies [11,13-15,23-25] provided the data on PD-L1 and OS in osteosarcoma. The pooled results were: HR=1.78, 95%CI=1.36-2.32, p < 0.001, showing that high PD-L1 expression associated with poorer OS (Table 2, Figure 2). To further investigate the relationship of PD-L1 and OS, subgroup analysis by ethnicity, sample size, and NOS score were conducted. The results showed that PD-L1 remained a significant prognostic marker regardless of ethnicity: Caucasians (HR=2.17, 95%CI=1.47-3.2, p < 0.001) or Asians (HR=1.48, 95%CI=1.02-2.14, p=0.039), sample size: for n <50 (HR=1.86, 95%CI=1.03-3.37, p=0.039) and n≥50 (HR=1.75, 95%CI=1.3-2.37, p < 0.001). However, PD-L1 had non-significant prognostic value for studies with NOS score ≤6 (HR=1.52, 95%CI=0.73-3.18, p=0.265) (Table 2 and Figure 2). Three studies [12,14,24] investigated the correlation of PD-L1 and DFS in osteosarcoma patients. The combined results were: HR=1.24, 95%CI=0.31-5.07, p=0.761 (Table 2; Figure 3).

PD-L1 and clinicopathological factors
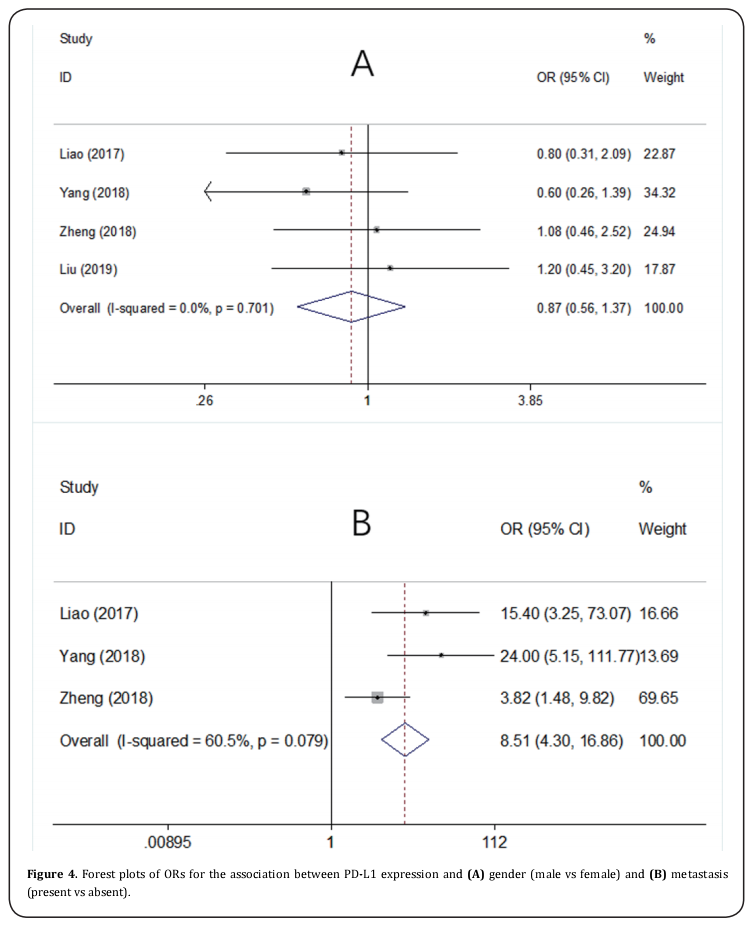
Four studies [14,22-24] presented the data on PD-L1 and clinicopathological factors including gender (male vs female) and metastasis (present vs absent). As shown in Figure 4, the pooled data were: OR=0.87, 95%CI=0.56- 1.37, p=0.556 for gender (male vs female), and OR=8.51, 95%CI=4.3-16.86, p <0.001 for metastasis (present vs absent).
Publication bias
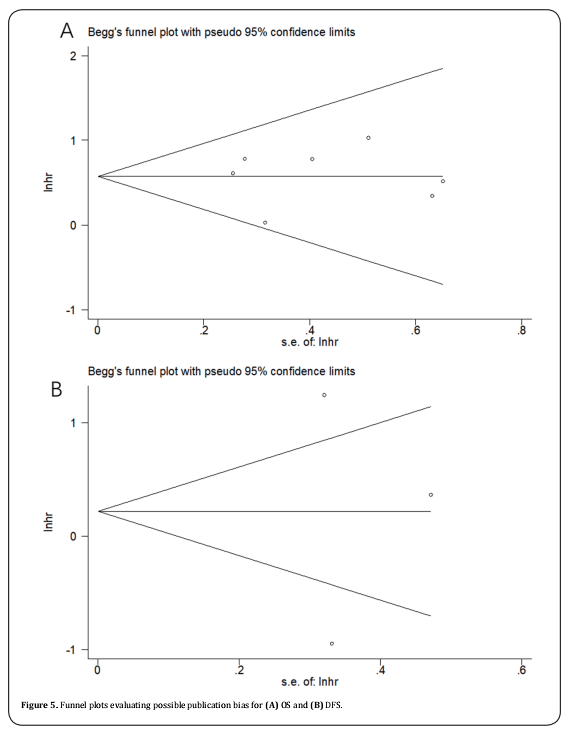
Funnel plots was used to evaluate publication bias. The funnel plots of the studies were symmetrical (Figure 5), showing no significant publication bias.
Discussion
The prognostic and clinical significance of PD-L1 in osteosarcoma were comprehensively analyzed based on data from 9 eligible studies. The aggregated results suggested that elevated PD-L1 expression predicted worse OS and DFS, irrespective of ethnicity and sample size. Furthermore, PD-L1 high expression was also correlated to positive metastasis status. Taken together, this study demonstrated that PD-L1 was a potentially prognostic marker for poor survival and tendency to metastasis in osteosarcoma.
PD-1 and its ligand PD-L1 are often overexpressed in tumor microenvironment, the biding of PD-1 and PDL1 can induce T cell apoptosis and IL-10 expression to negatively regulate the immune responses and result in immunosuppression [26]. The application of anti-PD-L1 antibodies also showed promising effects in different solid tumors [27-29]. Previous studies also showed the prognostic value of PD-L1 in diffuse large B-cell lymphoma [4], glioma [30], head and neck cancer [31], and nonsmall cell lung cancer [32]. Li’s study showed that positive expression of PD-L1 could serve as a good predictor for poor prognosis of Asian patients with head and neck cancer [31]. The results were in line with our study. Moreover, our data also showed the positive correlation of PD-L1 and metastasis in osteosarcoma. The finding may have clinical implications for osteosarcoma treatment.
First, the sample size was relatively small. Only 538 patients were included, which may influence the robustness of the statistical results. Second, all included studies were retrospective design. Third, only two clinical factors were investigated, which may ignore correlation of PD-L1 and other clinical characteristics.
In conclusion, this study demonstrated that PD-L1 high expression was associated with poor survival outcomes and positive metastasis status. PD-L1 expression is a significant adverse independent prognostic factor in osteosarcoma. However, due to several limitations, more prospective large-cohort studies are needed to verify these findings.
Competing Interests
The author reports no conflicts of interest in this work.
References
1. Wedekind, M. F., Wagner, L. M., and Cripe, T. P. (2018) Immunotherapy for osteosarcoma: Where do we go from here? Pediatric Blood & Cancer 65, e27227
2. Siegel, R. L., Miller, K. D., and Jemal, A. (2019) Cancer statistics, 2019. Ca-a Cancer Journal for Clinicians 69, 7-34
3. Meazza, C., and Scanagatta, P. (2016) Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Review of Anticancer Therapy 16, 543-556
4. Qiu, L. P., Zheng, H. L., and Zhao, X. Y. (2019) The prognostic and clinicopathological significance of PD-L1 expression in patients with diffuse large B-cell lymphoma: a metaanalysis. Bmc Cancer 19, 273
5. Hu, Y., Chen, W., Yan, Z., Ma, J., Zhu, F., and Huo, J. (2019) Prognostic value of PD-L1 expression in patients with pancreatic cancer: A PRISMA-compliant meta-analysis. Medicine (Baltimore) 98, e14006
6. Li, Y., He, M. Z., Zhou, Y. Y., Yang, C., Wei, S. Y., Bian, X. H., Christopher, O., and Xie, L. (2019) The Prognostic and Clinicopathological Roles of PD-L1 Expression in Colorectal Cancer: A Systematic Review and MetaAnalysis. Frontiers in Pharmacology 10, 139
7. Wang, C. J., Zhu, H. J., Zhou, Y. D., Mao, F., Lin, Y., Pan, B., Zhang, X. H., Xu, Q. Q., Huang, X., and Sun, Q. (2017) Prognostic Value of PD-L1 in Breast Cancer: A Meta-Analysis. Breast Journal 23, 436-443
8. Li, Y., Huang, Q. Y., Zhou, Y. Y., He, M. Z., Chen, J. H., Gao, Y. B., and Wang, X. (2019) The Clinicopathologic and Prognostic Significance of Programmed Cell Death Ligand 1 (PD-L1) Expression in Patients With Prostate Cancer: A Systematic Review and Meta-Analysis. Frontiers in Pharmacology 9, 1494
9. Li, J. H., Ma, W. J., Wang, G. G., Jiang, X., Chen, X., Wu, L., Liu, Z. S., Zeng, X. T., Zhou, F. L., and Yuan, Y. F. (2018) Clinicopathologic Significance and Prognostic Value of Programmed Cell Death Ligand 1 (PD-L1) in Patients With Hepatocellular Carcinoma: A Meta-Analysis. Frontiers in Immunology 9, 2077
10. Huang, L. J., Deng, X. F., Chang, F., Wu, X. L., Wu, Y., and Diao, Q. Z. (2018) Prognostic significance of programmed cell death ligand 1 expression in patients with ovarian carcinoma A systematic review and meta-analysis. Medicine 97, e12858
11. Shen, J. K., Cote, G. M., Choy, E., Yang, P., Harmon, D., Schwab, J., Nielsen, G. P., Chebib, I., Ferrone, S., Wang, X. H., Wang, Y. Y., Mankin, H., Hornicek, F. J., and Duan, Z. F. (2014) Programmed Cell Death Ligand 1 Expression in Osteosarcoma. Cancer Immunology Research 2, 690-698
12. Koirala, P., Roth, M. E., Gill, J., Piperdi, S., Chinai, J. M., Geller, D. S., Hoang, B. H., Park, A., Fremed, M. A., Zang, X., and Gorlick, R. (2016) Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci Rep 6, 30093
13. Arantes, D. A. C., Goncalves, A. S., Jham, B. C., Duarte, E. C. B., de Paula, E. C., de Paula, H. M., Mendonca, E. F., and Batista, A. C. (2017) Evaluation of HLA-G, HLA-E, and PD-L1 proteins in oral osteosarcomas. Oral Surgery Oral Medicine Oral Pathology Oral Radiology 123, E188-E196
14. Liao, Y., Chen, L., Feng, Y., Shen, J., Gao, Y., Cote, G., Choy, E., Harmon, D., Mankin, H., Hornicek, F., and Duan, Z. (2017) Targeting programmed cell death ligand 1 by CRISPR/ Cas9 in osteosarcoma cells. Oncotarget 8, 30276-30287
15. Palmerini, E., Agostinelli, C., Picci, P., Pileri, S., Marafioti, T., Lollini, P. L., Scotlandi, K., Longhi, A., Benassi, M. S., and Ferrari, S. (2017) Tumoral immune-infiltrate (IF), PD-L1 expression and role of CD8/TIA-1 lymphocytes in localized osteosarcoma patients treated within protocol ISG-OS1. Oncotarget 8, 111836-111846
16. Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Grp, P. (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Plos Medicine 6, e1000097
17. Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S., and Sydes, M. R. (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16
18. Stang, A. (2010) Critical evaluation of the NewcastleOttawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology 25, 603-605
19. Cochran, W. (1954) The combination of estimates from different experiments. Biometrics 10, 101–129
20. Higgins, J. P. T., and Thompson, S. G. (2002) Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539- 1558
21. Begg, C. B., and Mazumdar, M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088-1101
22. Yang, Z. J., Zhu, G. X., Yang, X., Zeng, K., Liu, F. X., and Sun, J. Y. (2018) Expression of PD-L1 associated with Ki-67 and chemotherapy response but not p53 in osteosarcoma. International Journal of Clinical and Experimental Medicine 11, 12571-12577
23. Zheng, B., Ren, T., Huang, Y., and Guo, W. (2018) Apatinib inhibits migration and invasion as well as PD-L1 expression in osteosarcoma by targeting STAT3. Biochemical and biophysical research communications 495, 1695-1701
24. Liu, P., Xiao, Q., Zhou, B., Dai, Z., and Kang, Y. (2019) Prognostic significance of PD-L1 expression and tumorinfiltrating lymphocytes in axial osteosarcoma. World neurosurgery
25. Yoshida, K., Okamoto, M., Sasaki, J., Kuroda, C., Ishida, H., Ueda, K., Okano, S., Ideta, H., Kamanaka, T., Sobajima, A., Takizawa, T., Kito, M., Aoki, K., Uemura, T., Haniu, H., Kato, H., and Saito, N. (2019) Clinical outcome of osteosarcoma and its correlation with programmed death-ligand 1 and T cell activation markers. Oncotargets and Therapy 12, 2513-2518
26. Errico, A. (2015) PD-1-PD-L1 axis: Efficient checkpoint blockade against cancer. Nature Reviews Clinical Oncology 12, 63
27. Mittal, D., Gubin, M. M., Schreiber, R. D., and Smyth, M. J. (2014) New insights into cancer immunoediting and its three component phases elimination, equilibrium and escape. Current Opinion in Immunology 27, 16-25
28. Powles, T., Eder, J. P., Fine, G. D., Braiteh, F. S., Loriot, Y., Cruz, C., Bellmunt, J., Burris, H. A., Petrylak, D. P., Teng, S. L., Shen, X., Boyd, Z., Hegde, P. S., Chen, D. S., and Vogelzang, N. J. (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558-562
29. Zou, W., Wolchok, J. D., and Chen, L. (2016) PD-L1 (B7- H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science Translational Medicine 8, 328rv4
30. Xue, S., Song, G., and Yu, J. M. (2017) The prognostic significance of PD-L1 expression in patients with glioma: A meta-analysis. Scientific Reports 7, 4321
31. Li, J., Wang, P., and Xu, Y. L. (2017) Prognostic value of programmed cell death ligand 1 expression in patients with head and neck cancer: A systematic review and metaanalysis. Plos One 12e0179536
32. Fan, Y. W., Ma, K., Hu, Y., Niu, W. X., Li, E. X., and Wu, Y. Y. (2017) Prognostic value of PD-L1 expression in non-small cell lung cancer: a meta-analysis. International Journal of Clinical and Experimental Medicine 10, 8735-8744